An enzyme is a catalyst protein that increases the speed of a chemical reaction by lowering the activation energy. A doubling the activation energy needed B cooling the reaction by 10C C doubling the enzyme concentration D increasing the concentration of reactants to 100 micromolar while reducing.
Solved How Does An Enzyme Work To Catalyze A Reaction Chegg Com
Activation energy is the energy required for a reaction to begin.
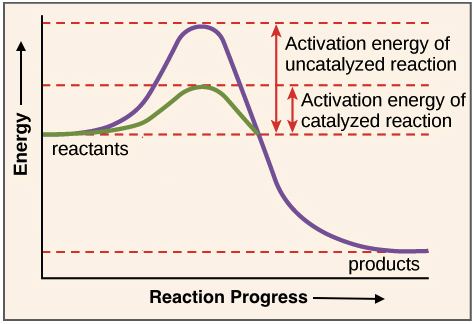
. When enzymes catalyze a reaction the preferred unit is the enzyme unit. Noun a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions as at a lower temperature than otherwise possible. For the enzyme-catalyzed reaction shown in the figure if the initial reactant concentration is 10 micromolar which of these treatments will cause the greatest increase in the rate of the reaction.
Two methods are generally used to prepare the enzymepolyNIPAM conjugates. Catalysis is a vital process in the chemical industry. This is a derived unit which is moles per second.
A introduction of polymerizable vinyl groups into the enzyme followed by copolymerization with NIPAM or b reaction of NH 2 groups on the surface of the enzyme with a copolymer of NIPAM containing reactive ester groups or the homopolymer containing an N-succinimide ester. The effectiveness of a catalyst may be expressed using the turnover number TON or turnover frequency TOF which is TON per unit time. It is estimated that 90 of commercially-produced.

Rate Of Reaction Enzymes Role Importance Expii

Managing Enzymes In Food Basics Of Enzyme Science Foodcrumbles

Enzyme Catalysis Introduction To Chemistry
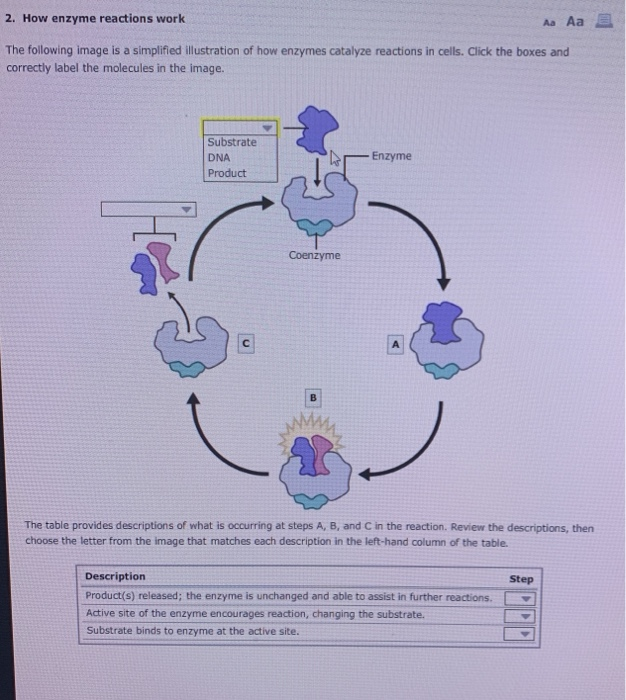
Solved 2 How Enzyme Reactions Work Aa Aa 3 The Following Chegg Com
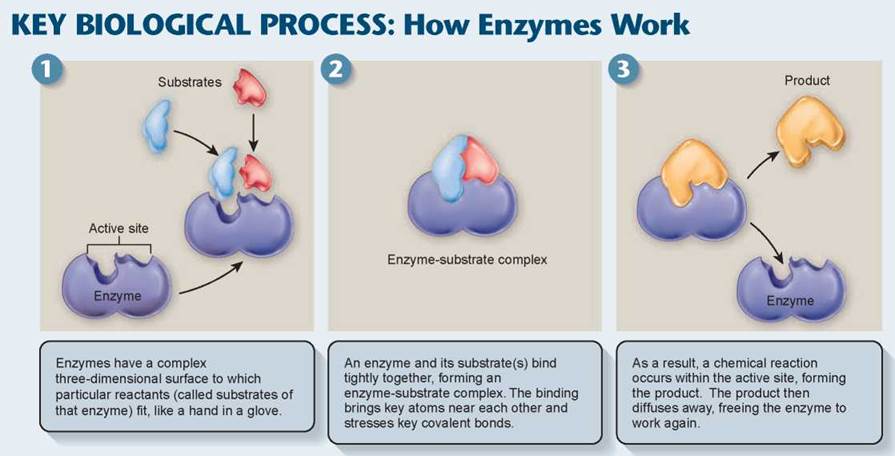
How Enzymes Work Energy And Life The Living Cell The Living World

Enzymes Review Article Khan Academy

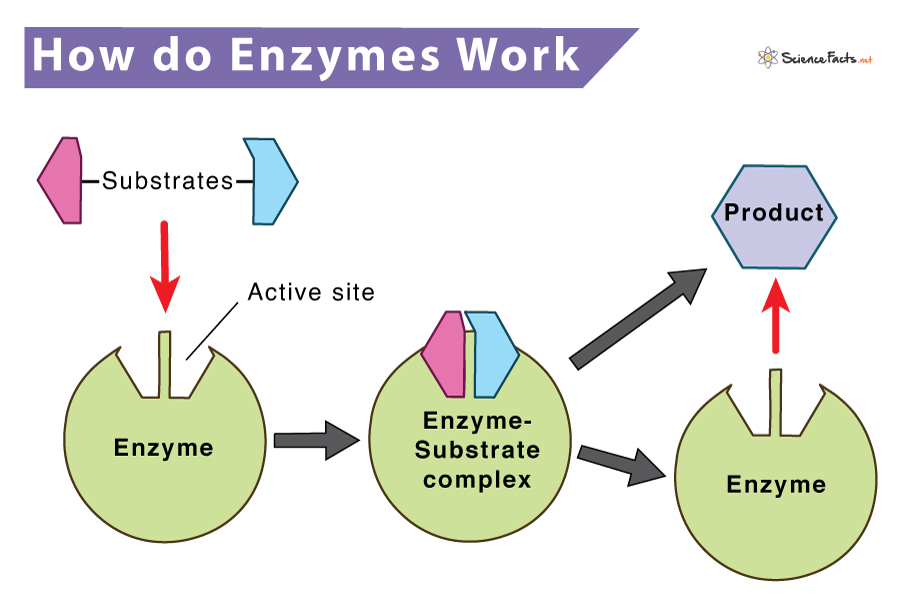
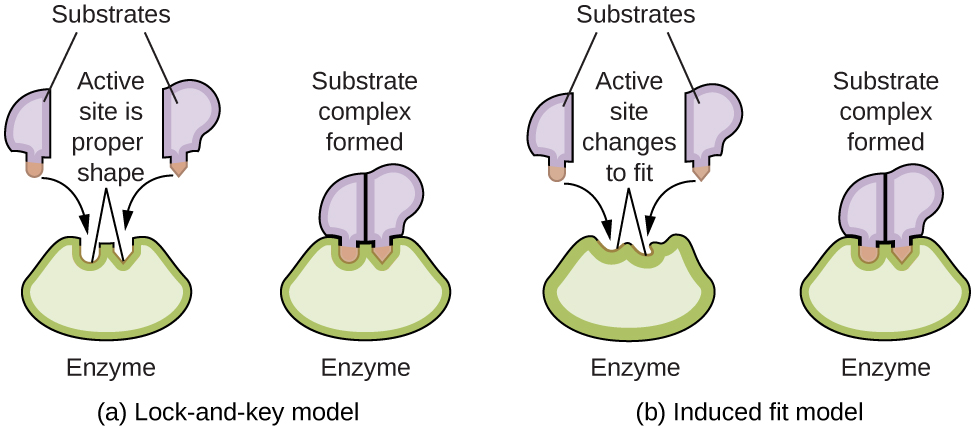
0 Comments